FDA Authorizes 1st At-Home Test For Both COVID & The Flu
Sick and wondering if you may have either COVID-19 or the flu? A new test cleared for use by the Food and Drug Administration promises to tell you just that.Thursday, March 2nd 2023, 8:49 am
Sick and wondering if you may have either COVID-19 or the flu? A new test cleared for use by the Food and Drug Administration promises to tell you just that.
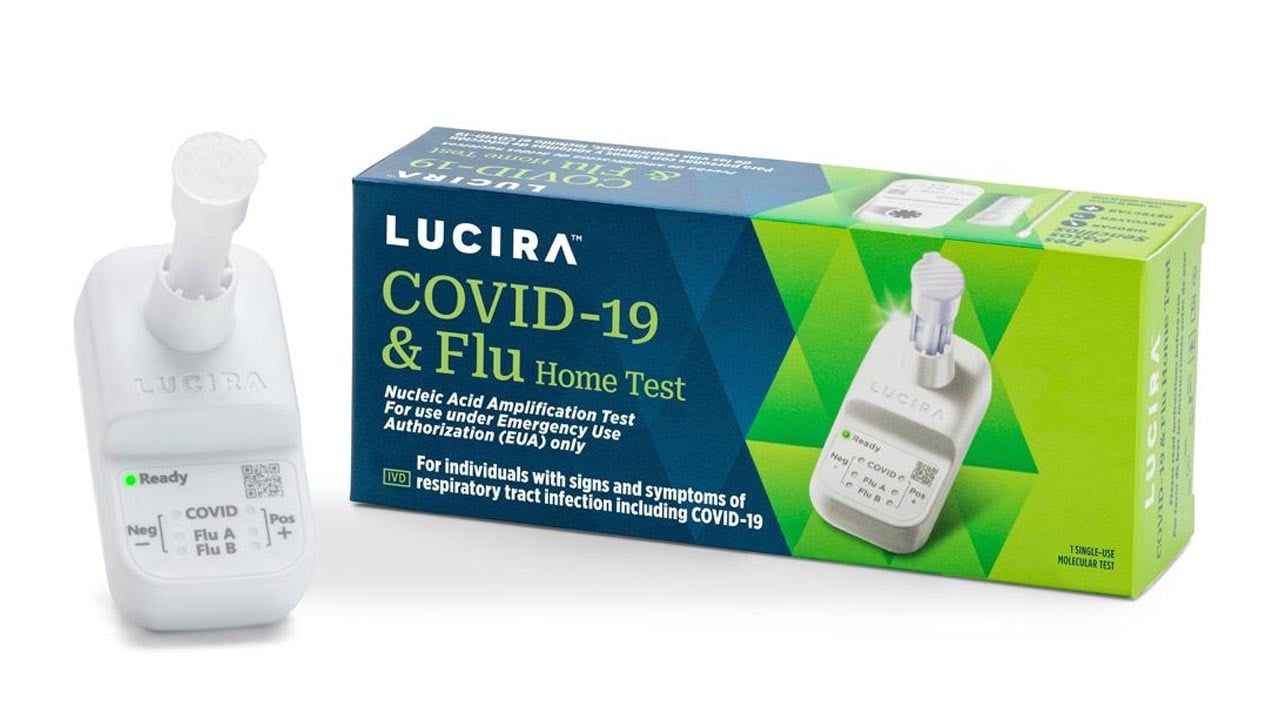
The Lucira COVID-19 & Flu Home Test is available over the counter and delivers results from a self-collected sample in roughly 30 minutes. The new test differentiates between COVID and the flu and indicates if a user is positive or negative for either of the viruses based on a nasal swab. It is the first such test to receive an Emergency Use Authorization (EUA) from the FDA.
"Today's authorization of the first OTC test that can detect Influenza A and B, along with SARS-CoV-2, is a major milestone in bringing greater consumer access to diagnostic tests that can be performed entirely at home," said Jeff Shuren, director of the FDA's Center for Devices and Radiological Health, in a statement.
The agency added that it is committed to expanding the availability of at-home tests that can detect contagious respiratory viruses.
Influenza A, B and COVID-19
The single-use diagnostic test from Lucira is designed for people with respiratory viral infection symptoms consistent with COVID-19 or the flu, and is suitable for anyone two years of age or older, according to the company.
The test works similarly to other kits approved for detecting COVID-19. Test users swirl the sample swab in a vial, which then goes in a test unit. The device delivers results indicating whether a person is positive or negative for influenza A, influenza B or COVID-19.
Without a diagnostic test, COVID-19 and the flu can easily be mistaken for one another.
"COVID-19 and flu look the same, feel the same, spread the same and, unfortunately, can still kill the same," said Dr. Davey Smith, head of infectious disease at the University of California – San Diego in a statement announcing the FDA's approval of the Lucira test kit. "Having an at-home molecular test now available should really help people know how to keep their families safe and seek appropriate treatment when they're ill."
Despite getting clearance from the FDA for its product, Lucira has struggled financially and filed for Chapter 11 bankruptcy protection only days before the agency authorized the test kit. That raises questions if the test will ever be widely available to consumers. For now, the kit is available for sale on Lucira's website for $99.
Lucira CEO Erik Engelson said in a statement that the company is seeking a potential strategic or financial partner to resume manufacturing and development of the product.
More Like This
March 2nd, 2023
March 23rd, 2023
February 7th, 2023
Top Headlines
December 14th, 2024
December 14th, 2024
December 14th, 2024
December 14th, 2024