FDA Sets New Mammogram Guidelines To Protect Women
The U.S. Food and Drug Administration has updated its mammography regulations, which affect physicians and medical centers across the country.Monday, March 13th 2023, 6:11 am
The U.S. Food and Drug Administration has updated its mammography regulations, which affect physicians and medical centers across the country.
As part of the new regulations, mammography facilities will have to notify patients about the density of their breasts. About half of American women over 40 have what's known as dense breasts. Having this type of tissue has been linked to a higher risk of developing breast cancer and can make it harder to diagnose the disease.
While similar laws have already been enacted in 38 states, including Colorado and Minnesota, this is the first nationwide regulation.
The new regulations will also "strengthen the FDA's oversight and enforcement of facilities and help interpreting physicians better categorize and assess mammograms," the agency said in a news release.
"Today's action represents the agency's broader commitment to support innovation to prevent, detect and treat cancer," said Dr. Hilary Marston, the FDA's chief medical officer. "Since 1992, the FDA has worked to ensure patients have access to quality mammography. The impact of the Mammography Quality Standards Act on public health has been significant, including a steep decrease in the number of facilities that do not meet quality standards. This means that more women have access to consistent, quality mammography. We remain committed to advancing efforts to improve the health of women and strengthen the fight against breast cancer."
What are dense breasts?
Dense breasts or dense breast tissue is the term used to describe the way some breasts look on a mammogram. Dense breasts have less fat and more tissue, so the images captured by standard mammograms may not show tumors or other masses. On mammographies, both masses and tissue show up as white.
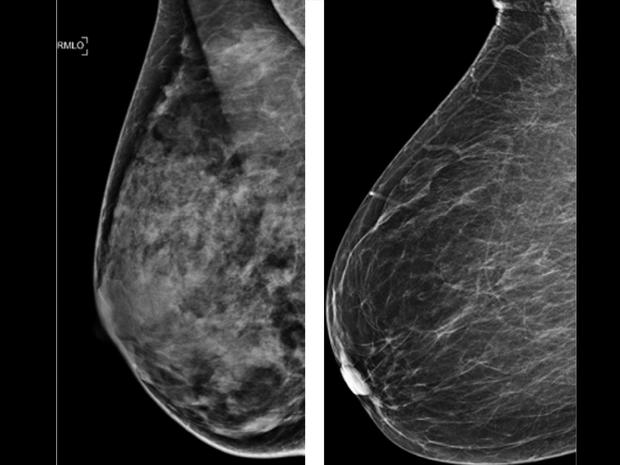
Dense breasts are very common, according to the National Cancer Institute, an entity of the National Institute of Health. About half of women over 40 in the United States who get mammograms have been found to have dense breasts. The condition is often inherited, but can also be influenced by using menopausal hormone therapy and having a low body mass index, according to the institute.
Dense breasts can't be determined by self-exams or clinical exams, only mammograms.
Having dense breasts has been linked to 1.2-4 times higher risk of developing breast cancer. However, according to the institute, there is no link between dense breasts and death from breast cancer. According to the FDA, 1 in 8 women will have breast cancer in their life.
What do the FDA changes mean?
The FDA changes are amendments to regulations issued under a 1992 law that gives the FDA oversight over mammography facilities, but it's not until now that the FDA has used the law to require facilities to inform women if they have dense breast tissue.
The requirements must be implemented within the next 18 months, the FDA said in a news release. The amendments provide "specific language explaining how breast density can influence the accuracy of mammography," and recommends that patients with dense breasts talk to health care providers about their individual situation and risk.
"While nearly all certified mammography facilities continue to meet quality standards, today's updates, among other things, enhance the FDA's ability to communicate directly, if needed, with patients and their health care providers in cases where a facility did not meet quality standards and is not adequately communicating with patients about its deficiencies," the FDA said in a news release. "This is intended to help ensure important information that could affect decisions about patient care, such as the potential need for further evaluation or a repeat mammogram, is communicated as completely as possible."
The FDA said the changes bring the 1992 law "into the 21st century" and use current science and mammography best practices to improve breast cancer detections and empower patients.
The changes do not mandate that mammography facilities use more intensive tools to detect breast cancer. People with dense breasts may use other imaging tests, like ultrasounds or MRIs, but the United States Preventative Task Force, an independent expert panel that issues screening recommendations, has said that it is not yet clear if the use of these additional tests is beneficial.
The FDA continues to recommend that people get regular mammograms. The task force advises that women aged 50 to 74 get a mammogram every other year. Younger women may also choose to get screened depending on their individual risk factors and family history.
More Like This
March 13th, 2023
November 18th, 2023
July 28th, 2023
Top Headlines
December 14th, 2024
December 14th, 2024
December 14th, 2024
December 14th, 2024